Avian Influenza
Avian influenza, sometimes avian flu, and commonly bird flu, refers to "influenza caused by viruses adapted to birds. Of greatest concern is highly pathogenic avian influenza (HPAI).
"Bird flu" is a phrase similar to "swine flu," "dog flu," "horse flu," or "human flu" in that it refers to an illness caused by any of many different strains of influenza viruses that have adapted to a specific host. All known viruses that cause influenza in birds belong to the species influenza A virus. All subtypes (but not all strains of all subtypes) of influenza A virus are adapted to birds, which is why for many purposes avian flu virus is the influenza A virus (note that the "A" does not stand for "avian").
Adaptation is non-exclusive. Being adapted towards a particular species does not preclude adaptations, or partial adaptations, towards infecting different species. In this way strains of influenza viruses are adapted to multiple species, though may be preferential towards a particular host. For example, viruses responsible for influenza pandemics are adapted to both humans and birds. Recent influenza research into the genes of the Spanish flu virus shows it to have genes adapted to both birds and humans; with more of its genes from birds than less deadly later pandemic strains.
Influenza pandemic
Pandemic flu viruses have some avian flu virus genes and usually some human flu virus genes. Both the H2N2 and H3N2 pandemic strains contained genes from avian influenza viruses. The new subtypes arose in pigs coinfected with avian and human viruses and were soon transferred to humans. Swine were considered the original "intermediate host" for influenza, because they supported reassortment of divergent subtypes. However, other hosts appear capable of similar coinfection (e.g., many poultry species), and direct transmission of avian viruses to humans is possible. The Spanish flu virus strain may have been transmitted directly from birds to humans.
In spite of their pandemic connection, avian influenza viruses are noninfectious for most species. When they are infectious they are usually asymptomatic, so the carrier does not have any disease from it. Thus while infected with an avian flu virus, the animal doesn't have a "flu". Typically, when illness (called "flu") from an avian flu virus does occur, it is the result of an avian flu virus strain adapted to one species spreading to another species (usually from one bird species to another bird species). So far as is known, the most common result of this is an illness so minor as to be not worth noticing (and thus little studied). But with the domestication of chickens and turkeys, humans have created species subtypes (domesticated poultry) that can catch an avian flu virus adapted to waterfowl and have it rapidly mutate into a form that kills in days over 90% of an entire flock and spread to other flocks and kill 90% of them and can only be stopped by killing every domestic bird in the area.
Until H5N1 infected humans in the 1990s, this was the only reason avian flu was considered important. Since then, avian flu viruses have been intensively studied; resulting in changes in what is believed about flu pandemics, changes in poultry farming, changes in flu vaccination research, and changes in flu pandemic planning.
H5N1 has evolved into a flu virus strain that infects more species than any previously known flu virus strain, is deadlier than any previously known flu virus strain, and continues to evolve becoming both more widespread and more deadly causing Robert Webster, a leading expert on avian flu, to publish an article titled "The world is teetering on the edge of a pandemic that could kill a large fraction of the human population" in American Scientist. He called for adequate resources to fight what he sees as a major world threat to possibly billions of lives. Since the article was written, the world community has spent billions of dollars fighting this threat with limited success.
H5N1
The highly pathogenic influenza A virus subtype H5N1 virus is an emerging avian influenza virus that has been causing global concern as a potential pandemic threat. It is often referred to simply as "bird flu" or "avian influenza" even though it is only one subtype of avian influenza causing virus.
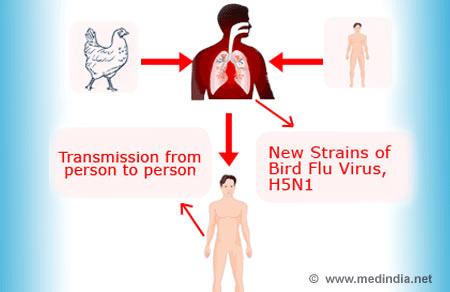
H5N1 has killed millions of poultry in a growing number of countries throughout Asia, Europe and Africa. Health experts are concerned that the co-existence of human flu viruses and avian flu viruses (especially H5N1) will provide an opportunity for genetic material to be exchanged between species-specific viruses, possibly creating a new virulent influenza strain that is easily transmissible and lethal to humans.
Since the first H5N1 outbreak occurred in 1997, there has been an increasing number of HPAI H5N1 bird-to-human transmissions leading to clinically severe and fatal human infections. However, because there is a significant species barrier that exists between birds and humans, the virus does not easily cross over to humans, though some cases of infection are being researched to discern whether human to human transmission is occurring. More research is necessary to understand the pathogenesis and epidemiology of the H5N1 virus in humans. Exposure routes and other disease transmission characteristics such as genetic and immunological factors, that may increase the likelihood of infection, are not clearly understood.
On January 18, 2009, A 27-year-old woman from eastern China has died of bird flu, Chinese authorities said, making her the second person to die this year from the deadly virus. Two tests on the woman were positive for H5N1 avian influenza, said the ministry, which did not say how she might have contracted the virus.
Although millions of birds have become infected with the virus since its discovery, 248 humans have died from the H5N1 in twelve countries according to WHO data as of January 2009. View the most current WHO Data regarding:Cumulative Number of Human Cases
The avian flu claimed at least 200 humans in Indonesia, Vietnam, Laos, Romania, China, Turkey and Russia. Epidemiologists are afraid that the next time such a virus mutates, it could pass from human to human. If this form of transmission occurs, another pandemic could result.
Thus disease-control centers around the world are making avian flu a top priority. These organizations encourage poultry-related operations to develop a preemptive plan to prevent the spread of H5N1 and its potentially pandemic strains. The recommended plans center on providing protective clothing for workers and isolating flocks to prevent the spread of the virus.
"Bird flu" is a phrase similar to "swine flu," "dog flu," "horse flu," or "human flu" in that it refers to an illness caused by any of many different strains of influenza viruses that have adapted to a specific host. All known viruses that cause influenza in birds belong to the species influenza A virus. All subtypes (but not all strains of all subtypes) of influenza A virus are adapted to birds, which is why for many purposes avian flu virus is the influenza A virus (note that the "A" does not stand for "avian").
Adaptation is non-exclusive. Being adapted towards a particular species does not preclude adaptations, or partial adaptations, towards infecting different species. In this way strains of influenza viruses are adapted to multiple species, though may be preferential towards a particular host. For example, viruses responsible for influenza pandemics are adapted to both humans and birds. Recent influenza research into the genes of the Spanish flu virus shows it to have genes adapted to both birds and humans; with more of its genes from birds than less deadly later pandemic strains.
Influenza pandemic
Pandemic flu viruses have some avian flu virus genes and usually some human flu virus genes. Both the H2N2 and H3N2 pandemic strains contained genes from avian influenza viruses. The new subtypes arose in pigs coinfected with avian and human viruses and were soon transferred to humans. Swine were considered the original "intermediate host" for influenza, because they supported reassortment of divergent subtypes. However, other hosts appear capable of similar coinfection (e.g., many poultry species), and direct transmission of avian viruses to humans is possible. The Spanish flu virus strain may have been transmitted directly from birds to humans.
In spite of their pandemic connection, avian influenza viruses are noninfectious for most species. When they are infectious they are usually asymptomatic, so the carrier does not have any disease from it. Thus while infected with an avian flu virus, the animal doesn't have a "flu". Typically, when illness (called "flu") from an avian flu virus does occur, it is the result of an avian flu virus strain adapted to one species spreading to another species (usually from one bird species to another bird species). So far as is known, the most common result of this is an illness so minor as to be not worth noticing (and thus little studied). But with the domestication of chickens and turkeys, humans have created species subtypes (domesticated poultry) that can catch an avian flu virus adapted to waterfowl and have it rapidly mutate into a form that kills in days over 90% of an entire flock and spread to other flocks and kill 90% of them and can only be stopped by killing every domestic bird in the area.
Until H5N1 infected humans in the 1990s, this was the only reason avian flu was considered important. Since then, avian flu viruses have been intensively studied; resulting in changes in what is believed about flu pandemics, changes in poultry farming, changes in flu vaccination research, and changes in flu pandemic planning.
H5N1 has evolved into a flu virus strain that infects more species than any previously known flu virus strain, is deadlier than any previously known flu virus strain, and continues to evolve becoming both more widespread and more deadly causing Robert Webster, a leading expert on avian flu, to publish an article titled "The world is teetering on the edge of a pandemic that could kill a large fraction of the human population" in American Scientist. He called for adequate resources to fight what he sees as a major world threat to possibly billions of lives. Since the article was written, the world community has spent billions of dollars fighting this threat with limited success.
H5N1
The highly pathogenic influenza A virus subtype H5N1 virus is an emerging avian influenza virus that has been causing global concern as a potential pandemic threat. It is often referred to simply as "bird flu" or "avian influenza" even though it is only one subtype of avian influenza causing virus.
H5N1 has killed millions of poultry in a growing number of countries throughout Asia, Europe and Africa. Health experts are concerned that the co-existence of human flu viruses and avian flu viruses (especially H5N1) will provide an opportunity for genetic material to be exchanged between species-specific viruses, possibly creating a new virulent influenza strain that is easily transmissible and lethal to humans.
Since the first H5N1 outbreak occurred in 1997, there has been an increasing number of HPAI H5N1 bird-to-human transmissions leading to clinically severe and fatal human infections. However, because there is a significant species barrier that exists between birds and humans, the virus does not easily cross over to humans, though some cases of infection are being researched to discern whether human to human transmission is occurring. More research is necessary to understand the pathogenesis and epidemiology of the H5N1 virus in humans. Exposure routes and other disease transmission characteristics such as genetic and immunological factors, that may increase the likelihood of infection, are not clearly understood.
On January 18, 2009, A 27-year-old woman from eastern China has died of bird flu, Chinese authorities said, making her the second person to die this year from the deadly virus. Two tests on the woman were positive for H5N1 avian influenza, said the ministry, which did not say how she might have contracted the virus.
Although millions of birds have become infected with the virus since its discovery, 248 humans have died from the H5N1 in twelve countries according to WHO data as of January 2009. View the most current WHO Data regarding:Cumulative Number of Human Cases
The avian flu claimed at least 200 humans in Indonesia, Vietnam, Laos, Romania, China, Turkey and Russia. Epidemiologists are afraid that the next time such a virus mutates, it could pass from human to human. If this form of transmission occurs, another pandemic could result.
Thus disease-control centers around the world are making avian flu a top priority. These organizations encourage poultry-related operations to develop a preemptive plan to prevent the spread of H5N1 and its potentially pandemic strains. The recommended plans center on providing protective clothing for workers and isolating flocks to prevent the spread of the virus.

1. Characteristics of candidate human H5N1 influenza vaccines
1.1. Safety
Q1.1 Are there important safety concerns about current H5N1 vaccines ?
Rationale: Human H5N1 vaccines are anticipated to be one of the major pharmaceutical
interventions against a pandemic of H5N1 influenza but have not been widely used in human
populations.
Evidence: Available data on the safety of H5N1 vaccines are relatively limited. However, no
evidence of any additional or unusual safety concerns related to H5N1 vaccines was presented.A recent consultation2 undertaken by the European Centre for Disease Prevention and Control (ECDC) on behalf of the European Commission (EC) identified no serious concerns in the publicly available data or in data disclosed confidentially by industry during the process. This is in line with presentations made at two WHO meetings on the clinical trial results of a range of pandemic candidate vaccines (including H5N1) in November 2005 and May 2006. None of the pandemic prototype vaccines tested were associated with additional safety issues and were well tolerated in the age groups studied.
There is no indication of any major differences in safety among current human H5N1 vaccines
regardless of whether antigen production is based on egg or cell-culture techniques, other than
potential allergic reactions to eggs which apply generally to all vaccines produced in eggs.
Additionally current data do not indicate any major safety differences among whole virus, split
or subunit vaccines, despite antigen presentation differences. Initial reports do indicate that
whole virus vaccines may be more reactive than split or subunit vaccines in terms of inducing
local (or minor systemic) reactions. Historical data indicate that whole virus influenza vaccines
in children were more reactogenic than subunit or split-virion vaccines.
Licensed inactivated subunit vaccines for seasonal influenza have been very safe and welltolerated;vaccines based on a new antigen from a pandemic influenza virus are considered
unlikely to pose unique risks. However, direct experience with the H5N1 virus as a vaccine
antigen is limited and there has been one clear association between the use of swine influenza
vaccine (H1N1 subtype) in 1976–77 with an elevated risk of Guillain-Barré Syndrome.
However, studies have shown no similarly strong association with any formulation used
subsequently.
Because H5 haemagglutinin appears to be generally less immunogenic than the haemagglutinin
of seasonal influenza A viruses, most human H5N1 influenza vaccines developed to date
contain an adjuvant. Use of such adjuvants to “potentiate” immune responses inherently
increases the level of uncertainty surrounding the risk of rare unanticipated adverse affects.
Novel adjuvants can provide a potent nonspecific immune and inflammatory stimulation in
addition to the simulation of the specific virus antigen in the vaccine, although the precise ways in which an adjuvant produces this effect is not fully understood. The presence of adjuvant may increase the frequency of local reactions (for example, redness, soreness or swelling at the injection site), and this has been observed with some newer oil-in-water emulsion adjuvants. It is unclear if these are correlated with any increased risk of rare adverse effects, but no major safety signals have been reported to date.
There is no current evidence of an increased risk of autoimmune conditions associated with such adjuvanted vaccines. However, there have only been limited controlled studies with long-term follow up and limited studies in children.
One concern that was discussed was the possibility that use of a live-attenuated vaccine based
upon an H5N1 virus in a non-pandemic situation could result in genetic reassortment between
the vaccine virus and a seasonal human virus. This potentially could lead to the creation of a
hybrid virus. The overall probability of such an event was considered to be low but not zero. It
was therefore considered prudent to avoid using live attenuated H5N1 vaccines during a non-pandemic period. There are only very limited reports of transmission of a live-attenuated seasonal influenza vaccine virus from a vaccinated child to another person.
Current considerations: At present, and based on the limited amount of safety data available,
there is no evidence of an unacceptable adverse event profile for any of the human H5N1
vaccines thus far evaluated.
Clinical trials involving human H5N1 vaccines offer important opportunities to capture essential safety data. However, the data produced by such trials are limited, and continuing efforts to confirm H5N1 vaccine safety after licensure and widespread administration are essential. If the decision is made to use H5N1 vaccines, then monitoring their safety profile will be key to ensure that any important or unexpected adverse events are identified rapidly so appropriate responses can be taken. Any proposal to use the current human H5N1 vaccines in a non-pandemic period, when human H5N1 influenza virus infections remain predominantly zoonotic, should also incorporate effective post-marketing surveillance for both safety and effectiveness.
Even if human H5N1 vaccines were shown to be safe in extensive clinical trials and in analysis
of large safety databases, it can be anticipated that additional safety signals may become
apparent with widespread use. Vaccine administration on the scale anticipated for a human
pandemic H5N1 vaccine, will likely lead to the reporting of adverse events, some of which
might be real and others of which might be only perceived, as well as possibly reports of
vaccine failures. Coincidental adverse events, unrelated to the use of vaccines, will almost
inevitably occur, and possibly at levels sufficient to raise concerns about their use.
The acceptability of the risk of adverse events, in the area where vaccine is deployed, will
depend largely upon when the vaccine is used and the associated perception of benefit. For
example, the level of risk of adverse events which would be considered unacceptable when
there is little or no disease, may well become more acceptable as disease levels rise, particularly if disease is associated with serious morbidity or death. As a result, the assessment of risk-benefit is a crucial aspect of determining the acceptability of human H5N1 vaccine deployment, and such assessments may well vary depending upon location and disease situation.
An increased frequency of local reactions and/or minor systemic reactions may occur with
adjuvanted or whole virus vaccines, compared to non-adjuvanted vaccines. However, different
adjuvants might exhibit different adverse-event profiles, and it must not be assumed that all
adjuvants will behave in the same way.
If an H5 pandemic develops, use of live attenuated influenza vaccines, based upon a pandemic
H5 virus, may be beneficial. Live attenuated vaccines have certain advantages in a pandemic
scenario. They might, for example, be produced more efficiently, and might also be more
immunogenic following a single application, than some other types of vaccine. However, in light of current knowledge, the use of a live attenuated vaccine, based on the H5N1 virus, in a
non-pandemic period when H5N1 viruses are not in wide circulation, may not be prudent. More studies are needed to guide usage of this type of H5N1 vaccine.
1.2. Immunogenicity
Q1.2 Are current H5N1 vaccines immunogenic ?
Rationale: In a period when human H5N1 infections occur infrequently, it is difficult to
demonstrate the effectiveness or efficacy of vaccine to protect against development of disease.
In this situation, assessing the capacity of candidate human H5N1 influenza vaccines to
stimulate the immune system is vitally important.
Evidence: Clinical trials indicate that H5N1 influenza vaccines differ from seasonal human
influenza vaccines in that the H5N1 vaccines generally elicit lower levels of immune response
at comparable antigen dosage levels in both human and animal models.
Based on current methods for assessing immunogenicity, the use of certain adjuvants appears to reduce the amount of antigen required to elicit the same immune response as higher dosages of the same antigen used without adjuvant. The ability of some adjuvants to reduce the amount of antigen needed has been termed "antigen sparing". Aluminium-containing adjuvants provide
less of this “antigen sparing” effect than oil-in-water emulsion adjuvants. Without adjuvant,
split and subunit influenza H5N1 virus vaccines are likely to require very high levels of HA
antigen per dose (up to 90 µg). There are indications that this may be reduced modestly through the use of aluminium-based adjuvants (to around 30 µg); and potentially even smaller HA antigen amounts appear to be immunogenic when used with newer oil-in-water emulsion
adjuvants (e.g., 7.5 µg with MF-59 and 3.8 µg with AS). The use of effective adjuvants in split
and subunit vaccines appears essential to minimize the amounts of antigen required to elicit an
immune response. There have been no human trials involving direct head-to-head comparisons
of adjuvanted and non-adjuvanted vaccines from different companies; such trials would enable
direct cross-comparison.
Although data are limited, adjuvanted vaccines that elicit higher titres of antibodies against
homologous challenge than non-adjuvanted vaccines, also appear to elicit higher titres of
antibodies against other H5N1 strains than the one used in the vaccine. In other words, the use
of adjuvants here may also broaden the heterologous response, which is a theoretical advantage, although the actual degree of clinical significance has not been established. The effect may depend upon the adjuvant used, with oil-in-water emulsion adjuvants stimulating higher responses than aluminium-based adjuvants against variant H5N1 strains. MF-59 is an oil-inwater emulsion adjuvant licensed for use in seasonal influenza vaccine in Europe. It has been tested in vaccines containing either H5 or H9 antigens, and has demonstrated an improved immune response profile with increased cross-reactivity to antigenic variants. Preliminary data in humans also indicate that when two doses of one H5N1 vaccine are used, administration of a third dose of a heterologous H5N1 vaccine 16 months or more can boost existing antibody levels. Once again this effect was more robust when an adjuvanted vaccine was used. These limited observations of vaccination in people are supported by animal data.
In addition to stimulating the antibody response, some adjuvanted human H5N1 influenza
vaccines may simulate cell-mediated immunity. A number of factors in cell-mediated immunity
are increased (including CD4+ cell; IFN; and IL-2 measures) with a constant increase across the range of antigen dosages tested. The possible clinical significance of these immunologic
observations is unknown.
Finally, available evidence indicates that inactivated whole H5N1 virus vaccines may be more
immunogenic than split or subunit vaccines. Data from one non-adjuvanted whole virus vaccine (using a wild-type H5N1 strain grown in cell culture) indicate that 2 doses of 7.5 µg per dose are immunogenic. Other whole virus vaccines formulated with aluminium-based adjuvants also appear promising; further studies are needed to determine the precise contribution of the adjuvant. The envelope and/or internal components of whole virus vaccines may contribute to vaccine immunogenicity in the same way as some synthetic adjuvants, as suggested by studies in mice. However, this has not been conclusively proven, or demonstrated in humans.
Current considerations: Broadly speaking, it appears that the use of certain adjuvants in
human H5N1 influenza vaccines can lead to a greater immunogenic response than that observed if the same vaccine does not contain an adjuvant. However, different adjuvants appear to confer differing degrees of immunogenicity, particularly when comparing the newer oil-in-water emulsion adjuvants with aluminium-based adjuvants. There is currently wide variability in the serological assays used to assess immunogenicity, and no agreed-upon immunological criteria or benchmarks for assessing the response to human H5N1 influenza vaccines. This lack of standardization in assay procedures is highlighted here as a priority issue. The European Medicines Agency (EMEA) Committee for Medicinal Products
for Human Use (CHMP) criteria3 for human seasonal influenza vaccines have been used in
Europe to assess human H5N1 and pandemic vaccines, but these may not be the most
appropriate criteria for use at the global level.
1.3. Cross-reactivity
Q1.3 Are current H5N1 vaccines broadly cross-reactive against nonhomologous
H5N1 viruses?
Rationale: H5N1 viruses have continued to undergo evolutionary genetic changes, and these
have resulted in antigenically heterogeneous and distinct clades, sub-clades and strains.
Currently ten clades have been identified. It is anticipated that further evolution and divergence will occur among these viruses and therefore the availability of H5N1 vaccines capable of inducing cross-reactive – and more importantly cross-protective – immunity against both currently circulating and future emerging strains, would be highly beneficial.
Evidence: Ten distinct clades of H5N1 virus have so far been recognized (0–9), based upon
ongoing phylogenetic analyses of the publicly available sequences of the haemagglutinin (HA)
gene of H5N1 viruses isolated from humans and animals. Sequences within a clade differ by less than 1.5%, while sequence differences of 1.5% or greater will place strains in different clades. The classification of viruses into clades is based upon genetic analyses, with the differences tending to reflect antigenic variations. However, it is important to note that the
antigenicity of influenza viruses is evaluated separately (for example, with hemagglutinination
inhibition (HI) panel testing and antigenic cartography) from genetic changes. Genetic changes
identified through sequencing do not necessarily indicate or predict the degree of antigenic
change. Clade 2 viruses are the most divergent, with five sub-clades (2.1–2.5) of which two (2.1and 2.3) are further delineated into more than one lineage. Viruses from clade 1 and from subclades 2.1, 2.2 and 2.3 have caused recognized human infection, and are used in a number of current candidate vaccines.
Ideally several vaccine candidate strains should be made available for several viruses from each clade to improve the likelihood that at least one of the candidates will be both immunogenic and protective, as well as optimal from a manufacturing perspective. Influenza H5N1 viruses isolated from animals have been used as vaccine candidates when an appropriate human isolate has not been available. The timely sharing of new H5N1 viruses with WHO and their rapid analysis are vitally important in ensuring that vaccine candidate strains are as up to date as possible.
Animal model studies have demonstrated the ability of H5N1 influenza vaccines to induce
protective levels of antibodies against homologous viruses, as well as cross-reactive immunity
to antigenically distinct H5N1 viruses. There are convincing data from mouse and ferret animal models indicating that some degree of cross-protection is possible, and that human H5N1 vaccines produced using virus from one clade might be protective against challenge by a virus from a different sub-clade or clade.
Preliminary studies in humans also indicate some degree of cross-reactivity in post-vaccination
serum samples to viruses representing different clades. Current data indicate that oil-in water
adjuvanted human H5N1 influenza vaccines elicit greater cross-reactive responses than nonadjuvanted
vaccines (with the possible exception of whole virus vaccines) both within and
across clades. There is no indication in the currently available data of any significant differences
in cross-reactivity among current human H5N1 influenza vaccines based on antigen production
in either egg or cell-culture techniques or means of antigen presentation. There are no data yet
available on differences between live-attenuated and inactivated H5N1 vaccines in terms of
cross-reactivity.
Current considerations: It is anticipated that if H5N1 evolves into a pandemic strain, then any
H5N1 vaccine stockpiled now during this non-pandemic period may not be a perfect antigenic
match. Furthermore, any pandemic virus will continue to evolve during the course of the
pandemic and later. In this situation, adjuvanted (or whole virus) vaccines may possibly confer
some heterologous cross-reactivity and cross-protection.
A high degree of cross-reactivity among vaccines made from current H5N1 virus clades could
indicate potential cross-reactivity with future antigenically drifted strains. However, there is no
certainty about the degree to which H5N1 vaccine made with current viruses will be able to
provide protection against future H5N1 viruses. It is likely that cross-reactivity will decrease as
the antigenic distance between current and future H5N1 strains increases due to viral evolution.
Some H5N1 strains appear to elicit higher antibody responses than others, and those that do,
often elicit response against variants. The data suggesting that cross-protective antibodies can be
generated is encouraging, but once again the effect may not be maintained as H5N1 viruses continue to drift.
Finally, the degree to which laboratory-demonstrated cross-reactivity may translate into crossprotection
in humans is uncertain. This will only become clearer once additional data from
studies become available, more widely divergent viruses are used and the results are correlated
with cross-immunity.
3. Options for using the WHO H5N1 vaccine stockpile
In March 2007, the participants of a High Level Meeting held in Jakarta, Indonesia called upon WHO to create a global stockpile of human H5N1 influenza vaccine. In April 2007, the WHO Strategic Advisory Group of Experts (SAGE) recommended to the Director General that there was sufficient evidence for WHO to create such a stockpile for use in countries without influenza vaccine production capacity and no ability to create national stockpiles. In May 2007, the Sixtieth World Health Assembly, in its Resolution WHA60.286, called for the Director
General:
… to establish, in close consultation with Member States, an international stockpile of vaccines for H5N1 or other influenza viruses of pandemic potential as appropriate, for use in countries in need in a timely manner and according to sound public-health principles, with transparent rules and procedures, informed by expert guidance and evidence, for operation, prioritization, release of stocks, management and oversight;
At the Pacific Health Summit held in June 2007 in Seattle, Washington, USA, GlaxoSmithKline Biologicals (GSK) announced that it would deliver 50 million doses of H5N1 adjuvanted human influenza vaccine over 3 years to support the WHO stockpile initiative. Assuming 2 doses per person, this amount could vaccinate 25 million people in the world’s poorest countries. Omninvest of Hungary, Baxter and Sanofi Pasteur have subsequently indicated their willingness to make H5N1 vaccine available for stockpiling.
At this time, a physical WHO stockpile of H5N1 vaccine does not exist, and its development will depend upon several factors, including discussions with manufacturers on the terms and conditions of their donation, as well as on technical issues such as obtaining further information on the stability of vaccines. Data from ongoing studies into the stability of GSK H5N1 vaccine are preliminary but current indications are that it will have a stability profile at least equivalent to seasonal vaccine.7 It will be incumbent on all producers providing vaccine for stockpiling to undertake extended stability testing for the purpose of identifying the longevity of both antigen and adjuvant (if presented separately).
This will be essential in establishing the supply replacement cycles for stockpiles. In addition, other issues such as the liability implications for any countries that might host or use the WHO international stockpile and stockpiles of supporting materials such as syringes must be investigated. Although development of the WHO H5N1 vaccine stockpile is under way, many of the specific issues related to its use are yet to be resolved. The most fundamental of these decisions is how the stockpile is to be used. This will then inform other issues such as its potential size, location(s), the triggers for release of vaccine, and stockpile management and governance processes.
Once recommendations have been developed on the basis of currently available scientific and other evidence, further issues such as liability, access protocols and logistical demands can be resolved. It is already apparent that the logistical challenges, both of maintaining and deploying a WHO H5N1 vaccine stockpile, are likely to be considerable, and these in themselves may limit its envisaged uses. To address these issues, WHO convened a second consultation from 17 to 19 October, to discuss the technical specifications required for an international H5N1 vaccine stockpile. The outcomes of those deliberations will be reported upon separately. At the consultation held from 1 to 3 October, experience in using other WHO vaccine stockpiles was reviewed.
Speakers stressed, however, that some of the “lessons learned” may not be directly applicable to the planned H5N1 vaccine stockpile, and cautioned against extrapolating more than is prudent. As a first step, two major options for the use of the WHO H5N1 vaccine stockpile were discussed:
3.1. To help contain the initial and localized emergence of a potential H5N1 influenza pandemic
Rationale: WHO has been planning how it would coordinate an operation to try and contain a potential pandemic of influenza, should the first outbreak be identified early enough to make the attempt feasible. The success of such an operation will depend upon the early detection and reporting of the first outbreak, and the implementation of multiple combined containment strategies. Recently, the potential use of human H5N1 vaccines has been discussed as part of
such containment strategies. If such vaccines are used in any attempt to stop a pandemic, then the capacity to mobilize vaccine supplies quickly enough to support such an operation, will be a critical component in their successful use in containment operations.
Current considerations: It is unlikely that a potential pandemic could be successfully contained through the use of vaccination alone. Vaccination, with some exceptions, is not normally used to “contain” outbreaks of seasonal influenza. Full immunity is likely to require two doses of vaccine, and to take 3 weeks to develop after the first vaccination. Moreover, the degree of antigenic match between stockpiled vaccine and a potential H5N1 influenza pandemic
virus cannot be known. There will also be considerable logistical challenges in vaccinating large numbers (possibly millions) of people over a relatively short period of time. Nevertheless, even with these considerations, mathematical modelling approaches suggest that under certain conditions, vaccination could make a significant addition to the effects of other control actions, by reducing the ability of the virus to spread through the populations immediately surrounding the containment zone. Preliminary models suggest that optimal benefit would be achieved if several million people could be vaccinated, but further modelling using
different assumptions will be needed to refine the estimates.
3.2. To provide countries least able to obtain H5N1 vaccines with some level of supplies following sustained human-to-human transmission of H5N1 influenza virus
Rationale: If efforts to contain the emergence of a potential influenza pandemic have failed or have not been attempted, and an H5N1 pandemic appears imminent, a global surge in vaccine demand is anticipated. In this situation, poorer and developing countries will be least likely to obtain vaccines unless there are dedicated supplies. These vaccines, once made available to these countries, could be used in a number of ways, including the protection of critical infrastructure personnel, as defined by the national authority concerned.
Current considerations: Efforts are under way to improve access to H5N1 vaccines so that ifan H5N1 influenza pandemic were to emerge, supplies of H5N1 vaccine will be more readily and widely available. Although such efforts will take time to implement, once a WHO stockpile is available, H5N1 vaccines could be made available to poorer and developing countries relatively rapidly. Eligibility criteria for receiving stockpiled H5N1 vaccines in this situation require definition, and recipient countries would need to decide how such vaccines would best be used.
---------------------------------------------------------------------------------------------------------
Short Quiz
1. What is avian flu?
ANS: It refers to influenza caused by viruses adapted to birds.
2. Pandemic flu viruses have some _____virus genes and usually some ____virus genes
A. horse flu, human flu
B. human flu, swine flu
C. avian flu, human flu
D. human flu, avian flu
ANS: C
3. When was the first H5N1 outbreak occurred?
A. 1996
B. 1997
C. 1998
D. 1999
ANS: B
Information prepared by Betty


0 comments:
Post a Comment